CDMO
We are a lentiviral vector GMP CDMO offering personalized solutions based on a complete adaptation to customer requirements.
VIVEbiotech offers a wide range of manufacturing-related services on the lentiviral vectors produced, such as:
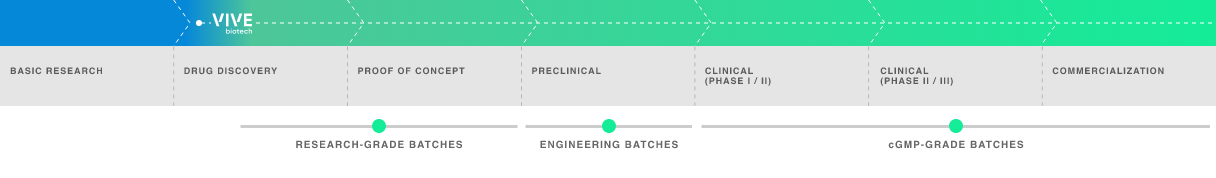
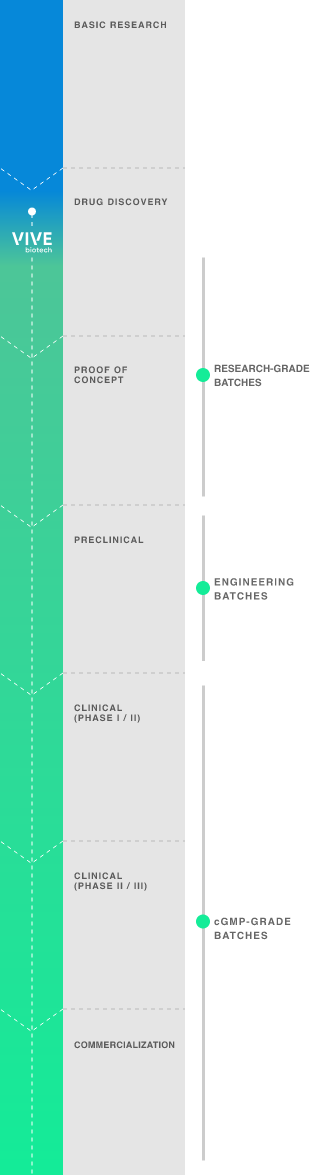
Early-phase development adapted to the requirements of each vector:
- Cloning activities: from design to cloning aimed at increasing productivity and conforming to regulatory requirements, among others.
- Various ad-hoc optimization activities: from adaptations in producer cell lines, to adjustments in transfection, to different pseudotyping strategies, etc.
- R&D manufacture in 2D format.
R&D manufacture in bioreactors.
Manufacture of engineering batches for preclinical phases.
GMP batches for clinical and commercial phases.
Batch release by our own Qualified Person.
Stability studies: long-term and in-use.
Regulatory support including:
- Chemistry, Manufacturing and Control (CMC) section of the Investigational Medicinal Product Dossier (IMPD).
- Chemistry, Manufacturing and Control (CMC) section of Investigational New Drug (IND).
Comprehensive management of outsourced partners (analytical testing and plasmid manufacturing, among others).
Specialization
Exclusively working in lentivirus.
GMP CDMO business model. Covering the different phases.
Meeting regulatory requirements. Compliance with EMA and FDA regulations.
Expertise in virology. We make scalable, cost-effective and regulatory compliant lentiviral vectors.
Customized approach to projets. Technical adaptation and slots availability in a timely manner.
Ability to adapt to different stages of development. Scales available for every stage, from early development to commercial manufacturing.
No own pipeline. Total priority to our customers' projects.
International company. Projects in the U.S., Europe, Asia and Australia.





